Greenhouse effect
Greenhouse gases are those whose increased concentrations lead to warming of the Earth's surface and the lower atmosphere, resulting in climate changes that negatively impact ecosystems. The mechanism, known as the 'greenhouse effect', is due to the following: solar radiation, which is in the visible spectrum (wavelengths of about 380 to 740 nm), passes through the atmosphere and reaches the Earth; most of the radiation is absorbed by the Earth's surface, seas and oceans, raising their temperature. Unabsorbed rays are emitted back from the Earth and water surface into the atmosphere, but in the infrared spectrum (wavelengths between 750 nm and 100 μm). Part of the infrared radiation passes through the atmosphere, while another part is absorbed by the greenhouse gas molecules and is emitted back in all directions. As a result, the Earth's surface and the lower layers of the atmosphere warm.
While the greenhouse effect has been one of the main prerequisites for the development of life on Earth for millions of years, raising its surface temperature and creating increasingly favourable conditions for the development of plants and animals, the last little over 100 years have seen the emission of greenhouse gases in greater quantities than nature can absorb. Over the last century, scientists have found that the earth has already warmed by about 0.5 oC. Based on projections of the increase in greenhouse gas emissions, the expected warming over the next 100 years will be in the range of 2-3 oC if no action is taken, especially if carbon dioxide concentrations double.
Major greenhouse gases
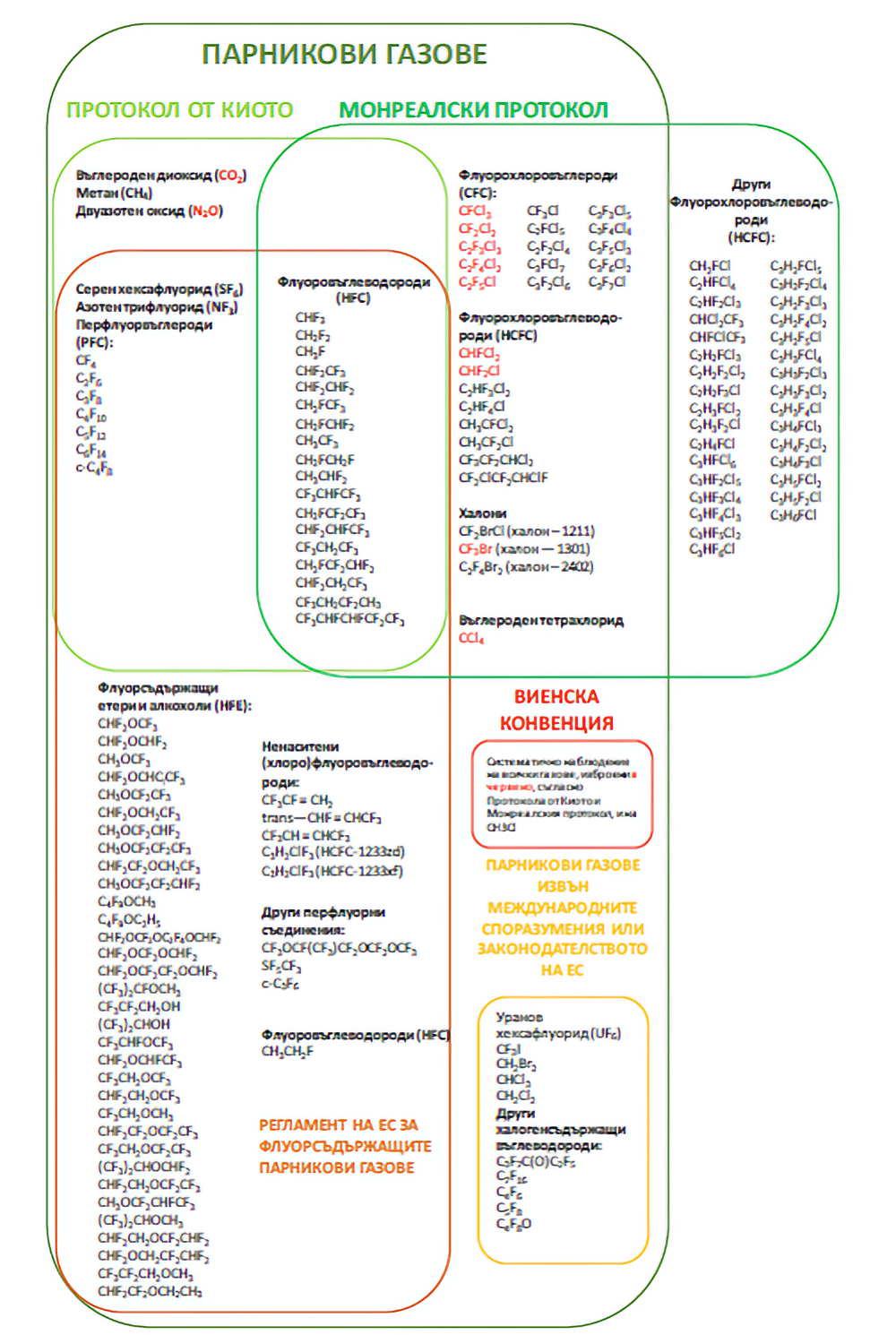
The main greenhouse gases that are most important in producing the greenhouse effect are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs) and sulphur hexafluoride (SF6). These are presented briefly below.
- Carbon dioxide (CO2)
Carbon dioxide is a colourless gas with a slightly sour smell and taste that dissolves well in water. It is emitted when people and animals breathe it, when fossil fuels (coal, oil and natural gas) are burned, as a result of power stations, etc.
The different greenhouse gases are recalculated as 'CO2 equivalent' because the impact each has on the climate is different (for example, one kilogram of methane has a significantly stronger negative effect than 1 kg of carbon dioxide). Carbon dioxide is the most important gas as it accounts for more than 80% of total greenhouse gas emissions in developed countries. Almost all CO2 emissions are due to the combustion of fossil fuels.
- Methane (CH4)
Methane is emitted from rice production, domestic livestock (e.g. cattle), landfill and municipal waste treatment, etc. It is a colourless, odourless gas which is highly flammable. It is also known as swamp gas as it is formed when biological waste decomposes in the absence of oxygen in swampy areas. Its concentration has doubled in the last three centuries and is expected to double again in the next 100 years. Methane is a major waste product of the microbial fermentation of biomass (plants) in the stomachs of ruminants (cattle, sheep, goats, etc.). Currently, for example, there are about 1.5 billion cows in the world, each of which releases between 70 and 120 kg of methane per year (through flatulence, from animal vomiting and from the anaerobic fermentation of manure). However, the negative effect of methane as a greenhouse gas is 23 times higher than that of carbon dioxide. Therefore, the release of about 100 kg of methane per year for each cow is equivalent to about 2 300 kg of CO2 per year.
- Nitrous oxide (N2O)
Nitrous oxide is emitted primarily from the use of fertilisers. As with methane, its emissions in developed countries have been stable or decreasing in recent years.
Emissions of nitrous oxide and methane are similar in that they are difficult to measure.
- Hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs)
These gases became popular in the 1990s as substitutes for some ozone-depleting substances that were used at the time in these applications, e.g., the fully halogenated chlorofluorocarbons (CFCs) and the incompletely halogenated chlorofluorocarbons (HCFCs) banned by the The Montreal Protocol. Although fluorinated greenhouse gases do not have ozone-depleting properties, most have a high global warming potential.
HFCs are the most common group of gases. They are used in a variety of industries and applications, as refrigerants in refrigerators, air conditioners and heat pumps, as blowing agents, fire extinguishing agents, aerosol propellants and solvents.
PFCs are commonly used in electronics (e.g. for plasma cleaning of silicon wafers), in cosmetics and pharmaceuticals (for extraction of natural products such as nutraceuticals and flavourings), and to a lesser extent in refrigeration as substitutes for CFCs - usually in combination with other gases. In the past, PFCs were used as fire extinguishing agents and are still found in older fire protection systems.
- Sulphur hexafluoride (SF6)
Sulphur hexafluoride is a colourless, odourless, non-flammable gas used in the semiconductor industry as a source of fluorine for high density plasma etching. It offers high selectivity without the formation of carbon by-products.
The gas finds application as an insulating gas and for arc extinguishing in high voltage switchgear and as a buffer gas in magnesium and aluminium production.
In electrical engineering, sulphur hexafluoride is used as a gaseous dielectric for high-voltage equipment - in circuit breakers, disconnectors, busbar systems and entire substations.
Other greenhouse gases
There are also a number of greenhouse gases that are exacerbating the greenhouse effect.
- Ammonia (NH3)
Ammonia enters the air from various sources - from livestock farming, from the soil and as a result of industrial activity. Around 90% of the pollution is due to agriculture, especially livestock farming. Ammonia is a by-product of animal waste, often due to the inefficient conversion of feed nitrogen to animal product. Ruminants reared for meat and milk, as well as poultry (hens, geese, etc.) are often fed on high-protein feeds that contain excess nitrogen to ensure that the nutritional requirements of the animals are met. Nitrogen that is not metabolized into animal protein (i.e., milk, meat, or eggs) is excreted in the urine and feces of livestock and poultry, where additional microbial action releases ammonia into the air during manure breakdown.
- Hydrocarbons
Hydrocarbons are organic compounds containing carbon and hydrogen atoms, e.g. methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), octane (C8H18) and their variants, e.g. ethylene (C2H4), propylene (C3H6), acetylene (C2H2), cyclohexane (C6H12), etc.
Hydrocarbons are released into the air when organic fuels (e.g. coal and oil) are burned and oil is refined. The main sources of pollution are the combustion of petrol in cars, livestock farming, natural sources and the domestic burning of organic matter.
- Ozone (O3)
Ozone is a form of oxygen with three oxygen atoms, and it has a light blue color and pungent odor. The gas is an unstable chemical and is a very strong oxidant.
The ozone layer in the Earth's atmosphere is the main "shield" that protects the Earth from dangerous ultraviolet solar radiation. The ozone absorbs entirely the most dangerous ultraviolet rays - the UVC rays that cause cancer in humans.
However, when we talk about air pollutants, we have to bear in mind that "useful" ozone is located in the upper atmosphere - 30 to 50 km above the Earth's surface. However, in the ground air layer, at altitudes up to about 10 km above the earth's surface, if present in high concentrations, it can have adverse effects. For example, ozone is a powerful oxidant that is formed by the interaction of nitrogen oxides and volatile organic compounds under the influence of high temperatures and sunlight.
- Carbon monoxide (CO)
Carbon monoxide is a toxic odourless colourless gas. It is produced by the incomplete combustion of coal, natural gas and wood materials.

